
The Enhanced Smallholder Livestock Investment Project (E-SLIP)

The Enhanced Smallholder Livestock Investment Project (E-SLIP)
The causative agent of ECF is Theileria parva, a tick-borne protozoan blood parasite. The life cycle of T. parva is complex in its tick and mammalian hosts (Fig 1).
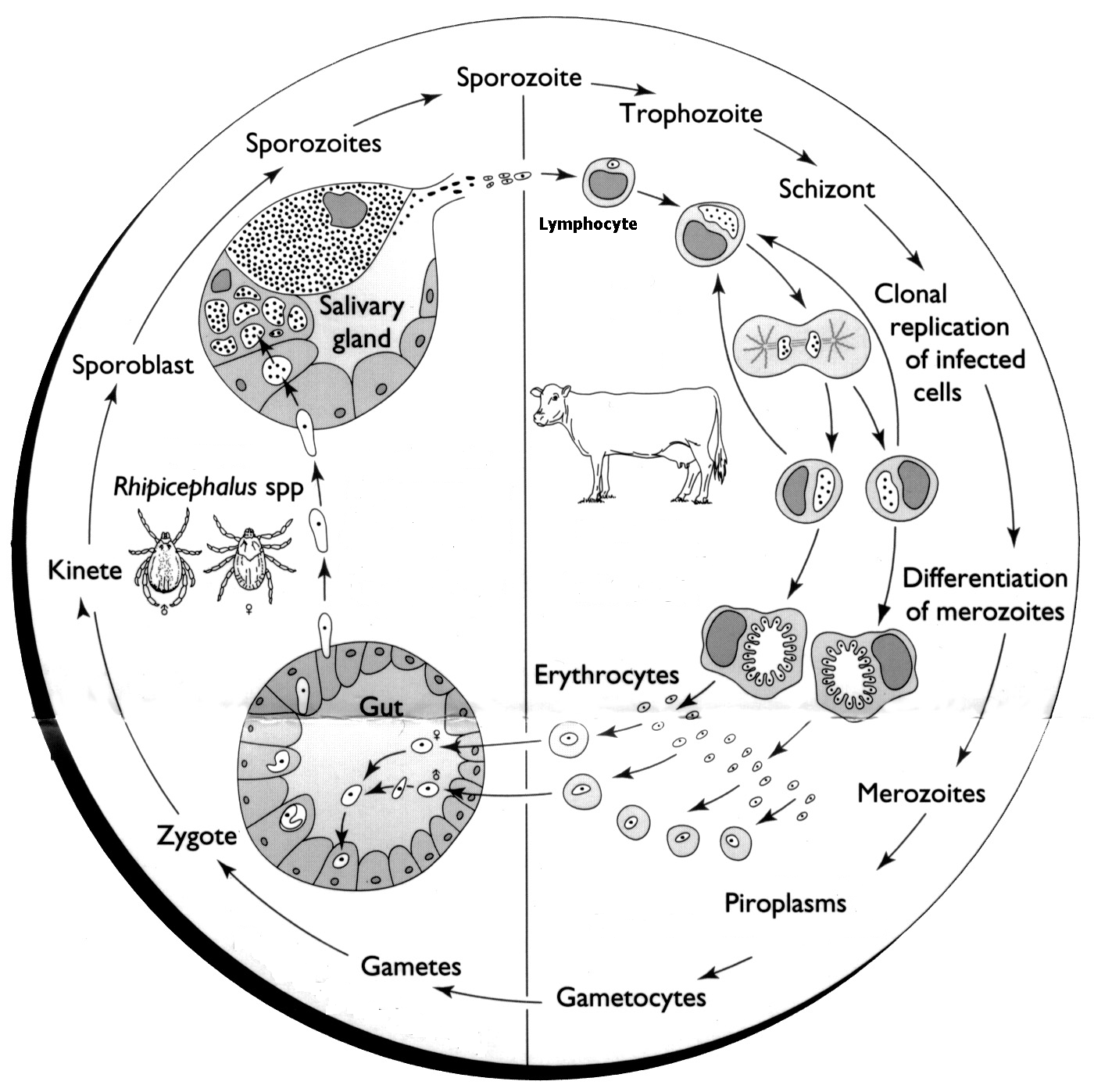
The vector R. appendiculatus is a three-host cattle tick with each stage (larva, nymph and adult) feeding on a separate animal. Theileria parva transmission is transstadial in that the larvae or nymphs that feed on infected bovine will transmit T. parva infection in their subsequent instars as nymphs or adults. There is no transovarial transmission.
During feeding, the infected tick will transmit sporozoites to a susceptible bovine through its saliva. Following infection, the sporozoites invade the T and B-lymphocytes of the host animal. The entry of the sporozoite into the lymphocytes involves a chain of events. Once inside the lymphocyte, the sporozoite develops into a schizont. Parasitised lymphocytes then transform to lymphoblasts, which have a capacity to divide repeatedly.
The schizonts develop into merozoites and the lymphocyte ruptures to release merozoites. These merozoites then invade erythrocytes and subsequently develop to piroplasms. It is the presence of these piroplasms in the infected animal that provide the source of infection for other ticks when they feed at the time of parasitaemia.
Theileria parva undergoes a sexual cycle in the tick to produce zygotes that invade gut wall cells. Thereafter, kinetes are produced which are capable of entering salivary gland cells. No subsequent development takes place until the tick starts to feed in its next instar. Sporogony occurs rapidly when the tick starts to feed and mature sporozoites that are infective to cattle are produced within the salivary gland cells three to five days later.
Cattle in endemic areas, particularly the zebu type (Bos indicus), appear less susceptible to ECF, as do young animals. In addition, introduced cattle, whether of a Taurine, Zebu, or Sanga breed, are more susceptible to ECF than cattle from endemic areas. The African buffalo (Syncerus caffer) is the reservoir of T. parva infection, and it has recently been proved that waterbucks (Kobus spp.) are also reservoirs. Therefore, wildlife plays an important role in the maintenance of the disease in the vector.
The distribution of ECF is strictly associated with the distribution of the vector tick species. The main vector, Rhipicephalus appendiculatus is found from sea level to over 2,400m above sea level in areas where there is annual rainfall of over 500 mm.
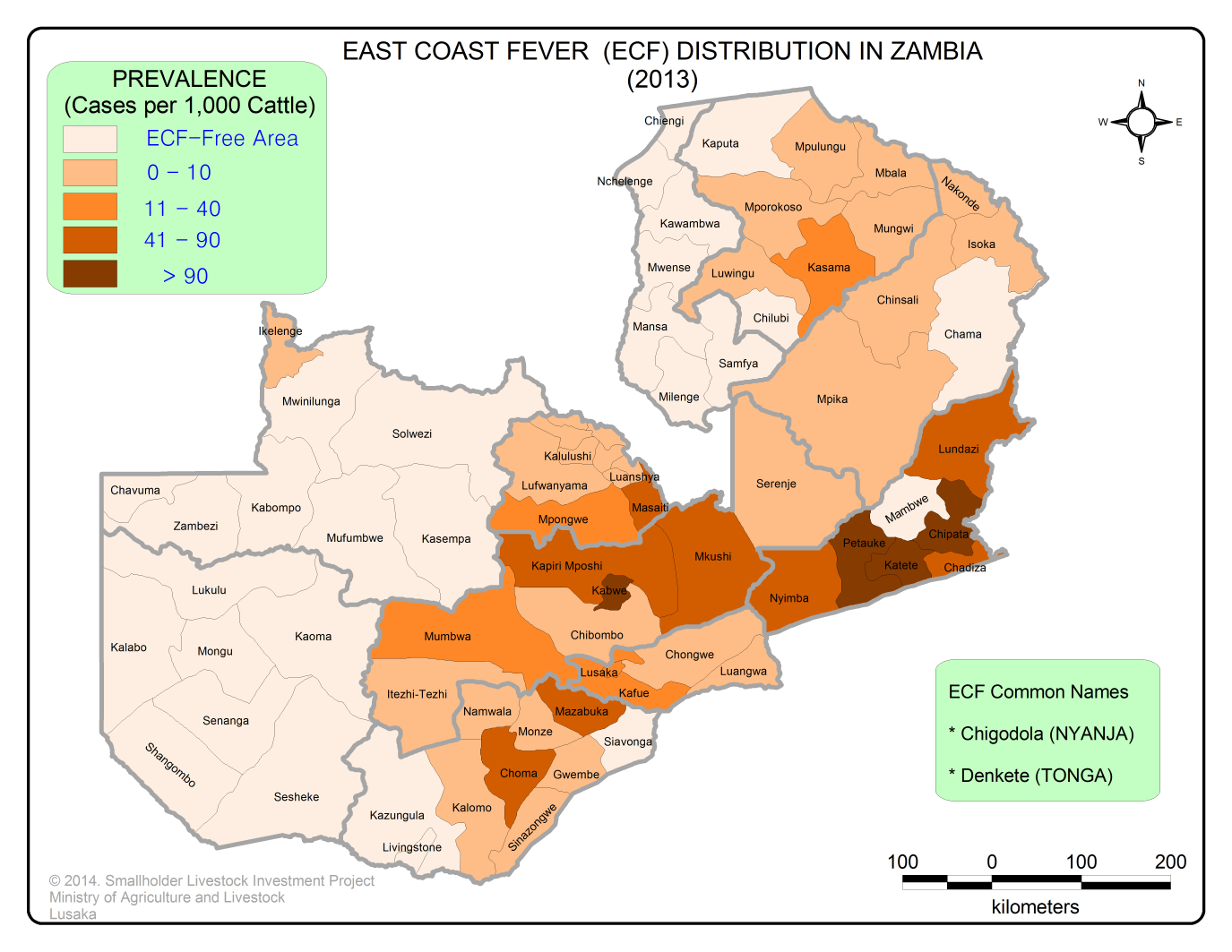
The distribution of the tick extends from southern Sudan to South Africa and as far west as Democratic Republic of Congo. The R. appendiculatus tick is found throughout Zambia. Other vectors of ECF include R. zambeziensis and R. duttoni. However, the range of T. parva is less than the tick vector. The distribution of T. parva is presented in the map below.
Rhipicephalus appendiculatus (Figs 3 & 4) is the main vector of ECF, although in drier areas of Southern Province other vectors, such as R. zambeziensis occur.




East Coast fever is not maintained in the absence of these vectors. The rhipicephalid vectors are three-host ticks, and transmission of the parasite occurs transtadially. Ticks can remain infected on the pasture for up to 2 years depending on the climatic conditions and the stage of infection. The parasite dies out faster in hot climates and in nymphs compared with adults. For transmission to occur, the infected tick has to attach for 3-7 days. During this time, sporozoites mature and are emitted in the saliva of the feeding tick. However, under high ambient temperatures, ticks on the ground may develop infective Theileria sporozoites, which can be transmitted to cattle within a few hours of attachment.
The incubation period in the field is dependent on the level of infected tick challenge on cattle and may extend beyond two (2) weeks after attachment of infected ticks. Under experimental conditions, using either ticks of known infection or sporozoite stabilate, the incubation period ranges from eight 8 to 12 days

Cattle that recover from ECF, either naturally or as a result of treatment with tetracyclines are solidly immune to homologous challenge. The immunity is mediated by cellular mechanisms targeted at the parasitised lymphoblasts. The major mechanism deployed by immune cattle against T. parva is the killer function of parasite-specific, major histocompatibility complex (MHC) class- I restricted CD8+ cytotoxic T-lymphocytes (CTLs).
There are four major ECF control measures currently available; namely tick control, chemotherapy, cattle movement control and Immunization (Infection and Treatment Method).
The main method of controlling ticks is the use of acaricides. The acaricides are applied onto cattle by means of dipping, which involves the animals plunging into the dip tanks or vats containing acaricide. The immersion of cattle during dipping ensures adequate exposure of ticks to the acaricide. The major challenges of dip tanks include the high cost of construction, the need for a steady supply of large volumes of water, the high cost of acaricide required for the initial charging of a tank and the requirement for a minimum number of cattle to use a dip tank in order to make its operation economically viable. To mitigate the startup costs, government is funding the construction, rehabilitation, and supply of initial acaricide.
Other means of acaricide application include spray race, hand-pump sprayer and pour-on. Spray races are an effective means of acaricide application. However, their effectiveness is reduced due to mechanical failure and lack of trained technical personnel. Hand-pump spraying rarely achieves complete wetting and result in poor tick control. It also tends to be uneconomical as excess acaricide which drips off the animals is not recovered. Acaricide impregnated ear-tags have also been shown to be effective in the control of ear ticks such as R. appendiculatus.
The Infection and Treatment Method (ITM) is used in the immunization against East Coast fever (ECF). The ITM uses cryo-preserved sporozoite stabilate made from T. parva-infected R. appendiculatus ticks. The stabilate is inoculated into the animal subcutaneously below the parotid gland and at the same time treated with long-acting tetracycline.
Immunization is only carried out in calves aged three (3) -18 months in endemic areas. Calves below three months are not immunized because of the interference of the maternal antibodies. Adults are not immunized as they are expected to have had contact with the parasite and hence acquired immunity that way.
Immunization against ECF is strain-specific, therefore, it is necessary to characterize the circulating strains in an area and conduct cross-immunity trials before embarking on immunization programs in such areas. This is to avoid the introduction of new strains into the chosen areas.
Immunization protects more than 70% of calves immunized. For this reason, it is advisable that the farmer immunises all calves in the herd. In addition, calves need to be immunized as early as possible within three (3)–18 months age bracket before exposure to the disease. Furthermore, immunizations are only done in ECF endemic areas.
There are a number of chemical products used in treating ECF. These include Parvaquone registered as Clexon®, Parvexon® and Parvexon Plus® (Bimeda), Buparvaquone registered as Butalex® (Pitman-Moore) and Halofuginone registered as Terit® (Hoechst).
For chemotherapy to be an effective method of control, it is important to have good diagnostic services. Further, drugs (theilericides) have to be available at all times since rapid intervention is required. Most of the chemotherapeutic agents are not effective once respiratory signs set in. After treatment and recovery, the animals become immune but also become carriers of the disease for life.
Restrictions on cattle movements can separate production areas from markets but are necessary to limit the spread of cattle diseases in general and ECF in particular. Once cattle come into contact with T. parva either through immunisation or natural infection, they become permanent carriers. Therefore, they are a permanent source of infection hence the need to restrict the movement of live cattle.